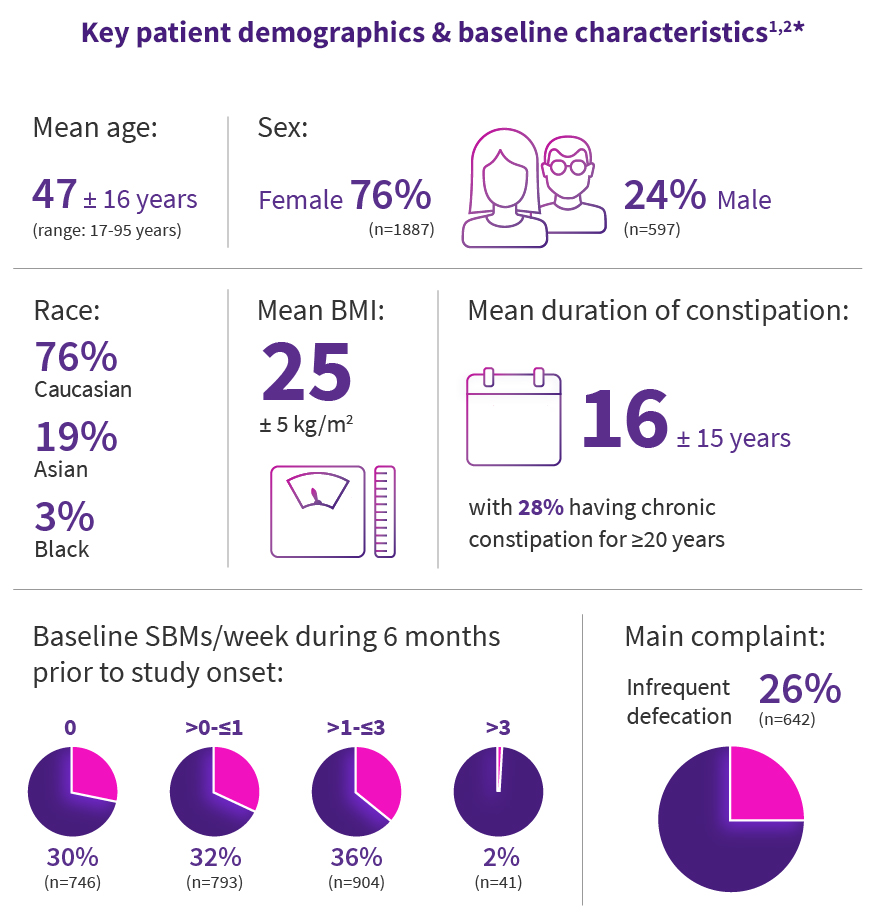
Study Design details1
In Studies 2 and 6, geriatric patients (≥65 years) started at 1 mg of Motegrity once daily and, if necessary, the dose was increased to Motegrity 2 mg after 2 or 4 weeks of treatment if response was insufficient at 1 mg. Of these patients, 81% (n=93/115) increased to 2 mg.1
| Inclusion Criteria1 | Exclusion Criteria1 |
|---|---|
|
|
|
|
|
|
|
| Inclusion Criteria1 |
|---|
|
|
|
|
| Exclusion Criteria1 |
|---|
|
|
BM=bowel movement; SBM=spontaneous bowel movement
Reference:
1.
Motegrity (prucalopride) Prescribing Information. Lexington, MA: Takeda Pharmaceuticals America, Inc.